Home > Resources > GCPPCS
Good Clinical Practice Professional Certification Scheme (GCPPCS) is first of its kind based on the International Personnel Certification Standard (ISO 17024:2012). GCPPCS is aimed to achieve an uniform, competence standard using internationally accepted best practice for assessment and certification.
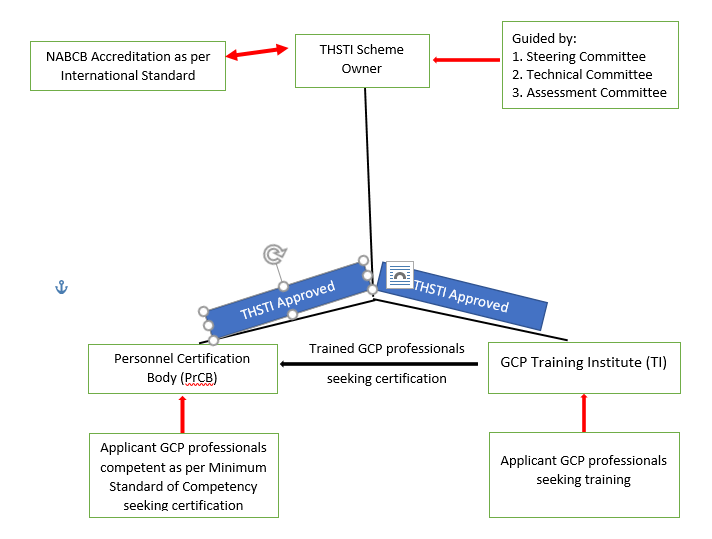
The GCPPCS is developed by the Translational Health Science & Technology Institute (THSTI). THSTI is an autonomous institution under the Department of Biotechnology, Ministry of Science & Technology, Government of India.
GCPPCS has two aspects – a system of accreditation of Training Institutions to ensure standardised, high-quality GCP training and certification (by Third-Party Certification) to promote certification of GCP professionals both within the country and across the globe.
There is an ever-growing demand for trained and certified Good Clinical Practice (GCP) professionals in the clinical research arena both in academia and in industry. THSTI as the Scheme Owner believes that GCPPCS will help in achieving a benchmark in the competence requirements.
The THSTI as the Scheme Owner defines the Minimum Standard of Competence and other requirements related for the certification of GCP professionals. THSTI is responsible for developing and maintaining the certification scheme. It has accredited Personnel Certification Bodies (PrCB) for conducting the certification process.
The PrCBs determines the fulfillment of the requirements for the certification process including application and assessment of the GCP professionals seeking certification, decision on certification, recertification and use of certificates and logos/marks.
The candidates who successfully demonstrate their competence to apply their knowledge and skills to achieve intended results during the assessment process are issued the certificate under the provision of international standard for a period of 5 years. During this period of 5 years the certified GCP professionals are under the surveillance of the PrCBs and the Scheme Owner.
Please refer to GCPPCS Documents to access and download the documents related to the certification of GCP Professionals
The THSTI as the Scheme Owner has defined the criteria for the assessment process and criteria for the provisional approval of the Training Institutions. It is responsible for maintaining the uniformity and standard of the Training Institutions as per Scheme requirements.
THSTI has accredited Training Institutions after conducting the assessment of the Training Institutions and witnessing the conduct of their GCP trainings.
The GCP professionals after successfully completing their training by the accredited Training Institutions would become eligible to apply for their certification by the accredited Personal Certification Bodies.
Please refer to GCPPCS Documents to access and download the documents related to the accreditation of Training Institutions.
Translational Health Sciences & Technology Institute, DBT, GOI, Faridabad, Haryana
Email: gcppcs.cdsa@thsti.res.in